
Hospital Disinfectant
Vital Oxide is a Fast and Effective Way to Kill Virus, Bacteria, and Mold That Cause Healthcare Associated Infections
Healthcare associated infections (HAIs) are the number four cause of death in the United States, exceeding the combined mortality of breast cancer, AIDS and traffic accidents. In a 2008 article, the editor of the New England Journal of Medicine, estimated that hospital acquired infections add an average of $15,000 to the cost of care which puts a total cost of infections in the U.S. around $40 billion a year.This cost is in addition to the suffering caused with more than 2 million infections a year, over 150,000 deaths and millions of extra days spent in the hospital.
VITAL OXIDE cleans, disinfects and deodorizes hard, nonporous hospital and medical surfaces in one step with no rinsing required.
VITAL OXIDE is a one step germicidal disinfectant cleaner and deodorant designed for general cleaning, and disinfecting of hard, nonporous inanimate surfaces, when use-directions for disinfection are followed. Removes dirt, grime, fungus, mold, food residue, blood and other organic matter commonly found in hospitals and in health care facilities. It also eliminates odors leaving restroom surfaces smelling clean and fresh. Use where odors are a problem. This product may be used to pre-clean or decontaminate critical or semi-critical medical devices prior to sterilization of high level disinfection.
CLEANING PROCEDURES: Blood and other body fluids must be thoroughly cleaned from surfaces and objects before application of this product.
--------------------------------------------------------------------------------
Nashville General Hospital Study - Acinetobacter
"The fogging of patient rooms of Acinetobacter infected patients with chlorine dioxide at discharge implemented at our hospital led to significant reductions in AcinetobacterHAI rates without the need for intrusive and costly additional interventions."
Incident of Acinetobacter infection in hospitals has dramatically increased in recent years becoming a significant global problem. (1.2)
Nashville General Hospital Study - Acinteobacter
These infections are often very difficult and costly to treat and have a mortality rate that approaches 75% in some settings (1, 2, 3). Moreover, Acinetobacter presents significant infection Control challenges since it may colonize both environmental surfaces as well as skin surviving for many months, may readily cause Hospital-Acquired Infections (HAI), is often resistant to multiple antibiotics, and often infects critically ill patients. (2,4,5,6). Accordingly, hospitals are often forced to take extensive and costly steps to prevent its spread that may be impractical in resource-limited settings. We present an easy to implement program for the reduction of Acinetobacter HAI rates in hospitals.
For this project we initiated a program in a 100-bed urban community teaching hospital in the U.S. whereby the Hospital Environmental Services Staff was notified immediately and automatically if any culture of any specimen taken from a hospital inpatient was found to be positive for Acinetobacter. Upon receiving this notification, the EVS staff would augment their standard terminal cleaning procedures by fogging the patient room with a chlorine dioxide solution (Figure 1) at the time of patient discharge in addition to their standard cleaning practices (Figure 2). We then reviewed the rates of infections meeting CDC NHSN definitions for HAI resulting from these pathogens for the 12 month period before and after the initiation of the intervention through active and passive surveillance of laboratory and other clinical records, coding data, and syndromic surveillance as well as the rate of community acquired laboratory-confirmed community acquired infections over this same time frame.
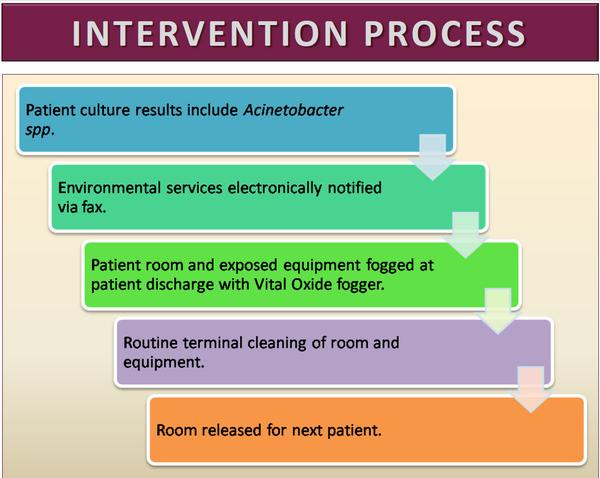
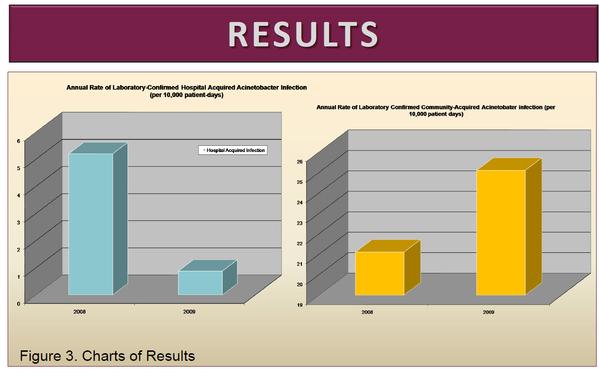
View source: Nashville General Hospital Study - Acinetobacter
In the 12 month period prior to the intervention, 13 Acinetobacter HAI were identified out of 25089 patient days for an aggregate rate of 5.2/10000 patient days (95% CI 3.0-8.9). For the period of the intervention only 2 Acinetobacter HAI were identified out of 22704 patient days for an aggregate rate of 0.88/10,000 patient days (95% CI 0.2-3.2) a decrease in Acinetobacter HAI Rate of 4.3/10000 (95% CI 1.1-8.0) Over the study period, the incidence of laboratory-confirmed community acquired Acinetobacter infection increased slightly from 4.42/month to 4.75/month.
Conclusion
The fogging of patient rooms of Acinetobacter infected patients with chlorine dioxide at discharge implemented at our hospital led to significant reductions in Acinetobacter HAI rates without the need for intrusive and costly additional interventions. Nashville General Hospital Study - Acinetobacter
Heading
To add this web app to your homescreen, click on the "Share" icon
![]()
Then click on "Add to Home"
To add this web app to your homescreen, click on the "Share" icon
![]()
Then click on "Add to Home"

It looks like your browser doesn't natively support "Add To Homescreen", or you have disabled it (or maybe you have already added this web app to your applications?)
In any case, please check your browser options and information, thanks!
It looks like your browser doesn't natively support "Add To Homescreen", or you have disabled it (or maybe you have already added this web app to your applications?)
In any case, please check your browser options and information, thanks!